A chance for a change
U.S. announces COVID-19 vaccine trials
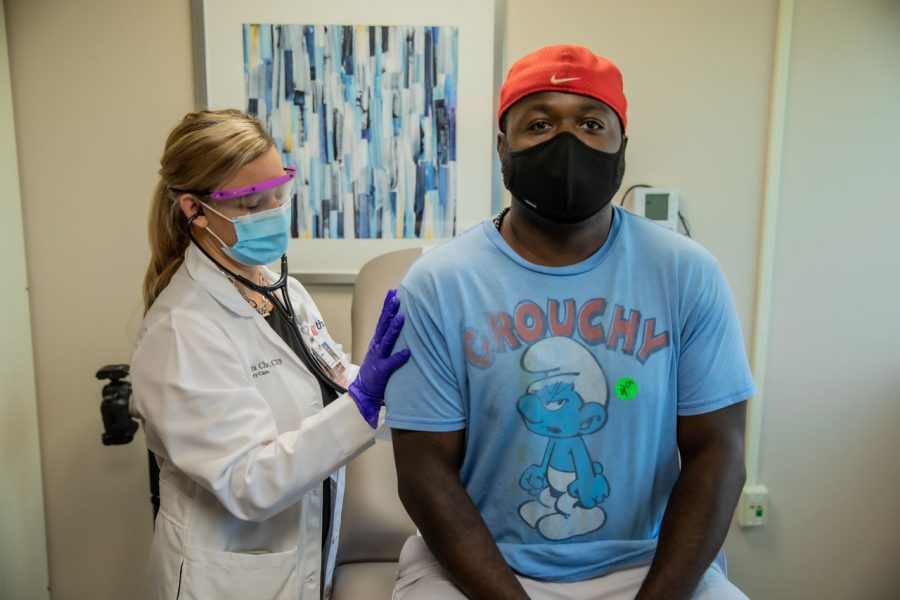
Photo courtesy of ScienceMag.org
This Ohio man was among the large number of people who received and was involved in Moderna’s COVID-19 clinical trial that showed a 94.1% effectiveness rate.
December 2, 2020
Taking over our lives in 2020, COVID-19 has changed our way of living in many drastic ways. Not only has this been a rough patch for everyone, but especially for the lives of the elderly and those with pre-existing conditions. However, thanks to many brilliant doctors and researchers, there may be hope for a future vaccine.
As of right now, there are no actual authorized vaccines to prevent COVID-19. However, the United States has begun its work in producing and partaking in different trials for different immunizations.
According to HHS.gov, “The U.S Department of Health and Human Services released two documents outlining the Trump Administration’s detailed strategy to deliver safe and effective vaccine doses to U.S citizens as quickly as possible.” The documents provide an overview and outline for the necessary steps to take for the COVID-19 program. Stated within are four required tasks: engage with the state and public to communicate public health information, distribute vaccines immediately upon authorization, ensure safe administration of the vaccine and monitor data through the vaccine program through an information technology system.
Currently, there are five different vaccines being tested in large-scale clinical trials. AstraZeneca, Janssen, Moderna, Novavax and Pfizer are all pharmaceutical companies that are in the process of creating these immunizations for the U.S. According to ScienceMag.org, “the biotech company Moderna announced the final results of the 30,000-person efficacy trial for its candidate: Only 11 people who received two doses of the vaccine developed COVID-19 symptoms after being infected with the pandemic Coronavirus, versus 185 symptomatic cases in a placebo group.” Moderna is now seeking emergency F.D.A approval for this vaccine to be put into place. “If authorization is granted, the first shots could be given as early as Dec. 21,” The New York Times stated on November 30.
Although a vaccine might be a possibility in the near future, the amount of doses will be limited. Supplies will be administered to certain groups who may be at a higher risk first to help reduce less deaths and/or cases. According to the CDC, “ACIP is considering four groups to possibly recommend for early COVID-19 vaccination if supply is limited; healthcare personnel, essential workers, people 65 years of age or older and people with severe underlying medical conditions.” These conditions include but aren’t limited to, Cystic Fibrosis, Asthma, liver disease and Neurologic conditions.
The safety and accuracy of this new immunization is top priority for those all involved with finding it. If a vaccine is authorized soon within the U.S, researchers hope to have a solid amount of doses for the public in early spring of 2021. Although masks and social distancing are two of the best ways to fight this pandemic, a vaccine is the only real way out of this.